HESI Innovation Prize
Now Accepting Applications for TWO Awards!

The 2022-2023 HESI Innovation Prize will recognize public sector scientists who have helped to reduce disproportionate health burdens in underserved populations and/or to promote greater health equity. Both Mid-career ($75,000 USD) and Lifetime Achievement ($25,000 USD) awards will be offered.
Interested in applying? Click here!
Applicants must submit a short letter (maximum 5 pages) that includes examples of the impact of the nominee’s work in creating novel, cross-disciplinary collaborations in relation the year’s thematic focus. Applications must also include a CV (5-10 pages maximum) for the nominee, as well as 1-2 letters of support.
Applications are being accepted from October 1 – December 15, 2022
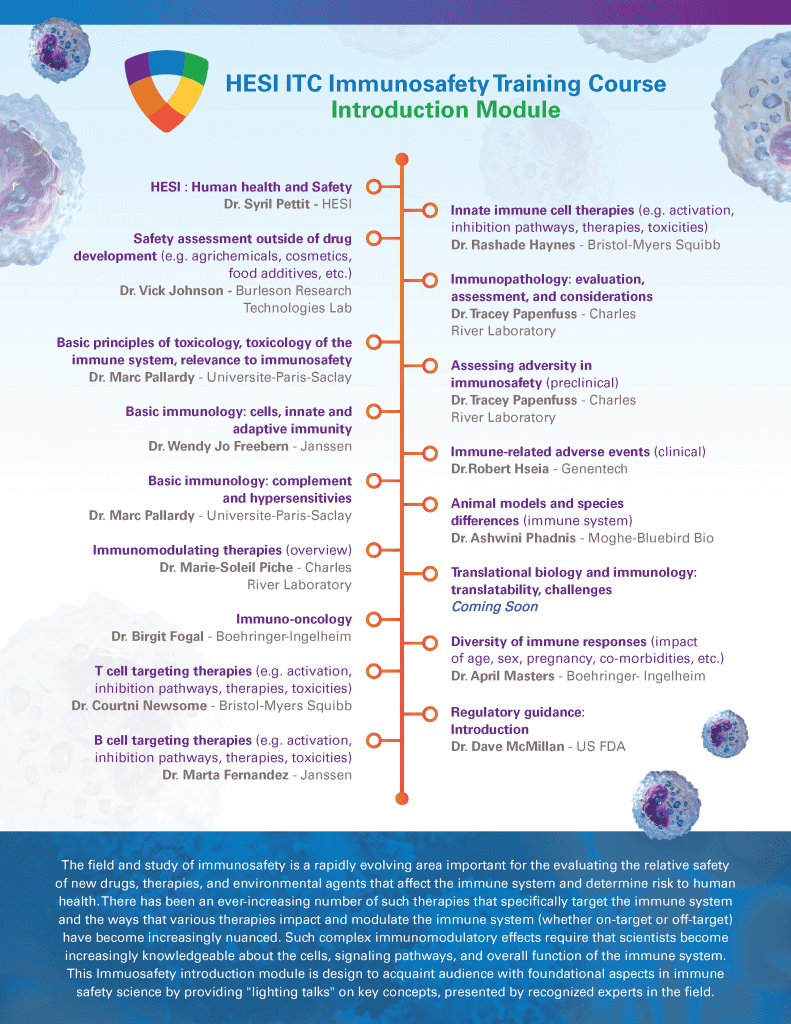
Immuno-Safety Training Course
Now Live!

Register now for the Immuno-Safety Technical Committee’s “Introduction to Immuno-safety Science” on-demand training course module.
This module features a collection of 16 lightening talks (~10-20mins) designed to introduce you to key concepts and tools that are essential to mastering fundamental knowledge within this discipline. All together this course will provide a comprehensive overview of the field in “bite size” digestible pieces suitable for professionals who need refresher or quick snapshot of the immuno-safety landscape to ground them in human risk and safety assessment as it pertains to agents that may modify the immune system. Register now and begin taking the course today at https://elearning.hesiglobal.org/ . Tiered pricing is available for graduate and post-doctoral trainees. Groups of 5 or more are eligible for a 20% discount, please contact Dr. Shermaine Mitchell-Ryan (smitchell-ryan@hesiglobal.org) for more information regarding the group discount rate. An Advanced course in Cancer Immunotherapies will be released in November!
Welcome new (sort of) HESI Staff
Raegan O’Lone

Please join us in welcoming Dr. Raegan O’Lone back to the HESI staff as a Senior Program Advisor! Dr. O’Lone will work with Dr. Lucilia Mouriès on the Targeted Protein Degrader Safety Committee and other current/evolving projects and activities.
Dr. O’Lone was an Associate Director at HESI when she departed in 2017 after moving from the DC area. She started with HESI in 2008 and provided outstanding scientific and organizational leadership across a broad range of programs including the Immunotoxicology and Genomics Committees. In the intervening years, she has gained novel experiences working with academic and nonprofit institutes focused on clinical and biotech portfolios.
We are excited to welcome her back to the team (and to the DC area)! The Senior Program Advisor is a new staff role at HESI and will provide technical and strategic leadership to specific committees across our scientific programs, and in the development of novel programs.
Welcome back!
HESI collaborates to help identify alternatives to animal testing

The Health and Environmental Sciences Institute (HESI) is excited about the recent news on the passing of the FDA Modernization Act 2.0 which will allow for the use of methods other than animal testing to establish a drug’s safety and effectiveness. These methods have the potential to both reduce animal use and lower the cost of developing life-saving medicines. The U.S. Food and Drug Administration (FDA) has acknowledged HESI’s role in this important endeavor and we are honored to be recognized for taking part in the 3Rs (Replacement, Reduction and Refinement) of animals in research by the FDA and many other leading safety standard organizations (e.g. OECD, EPA, JRC, etc.).