Impact of HESI Publications
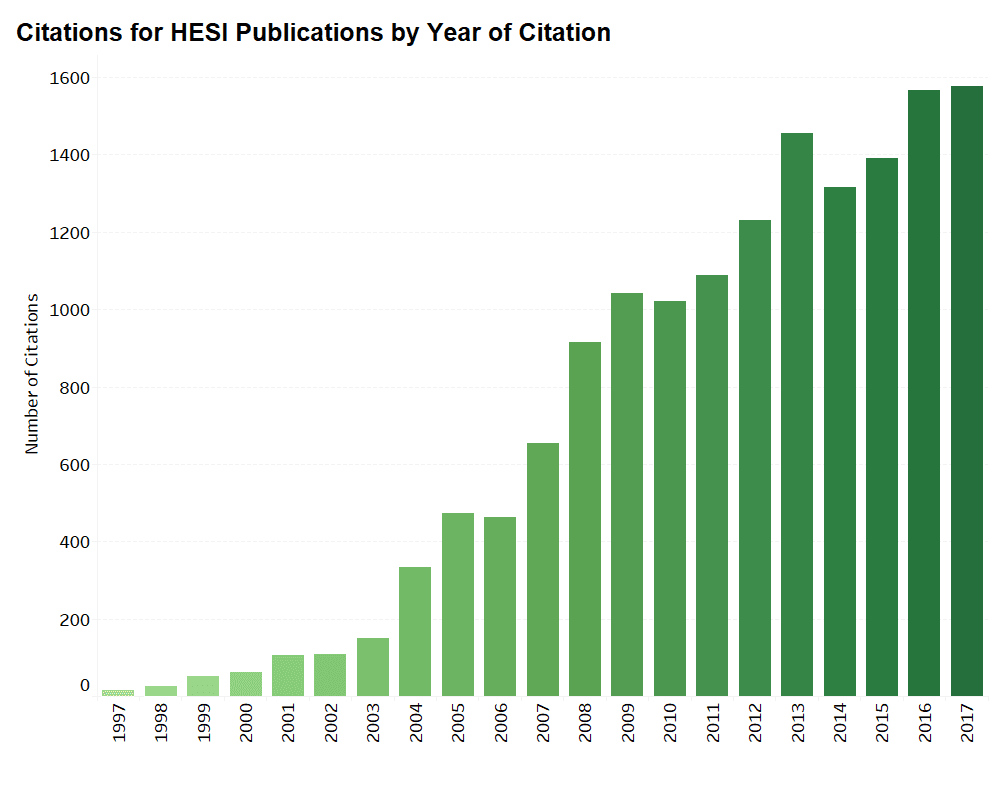
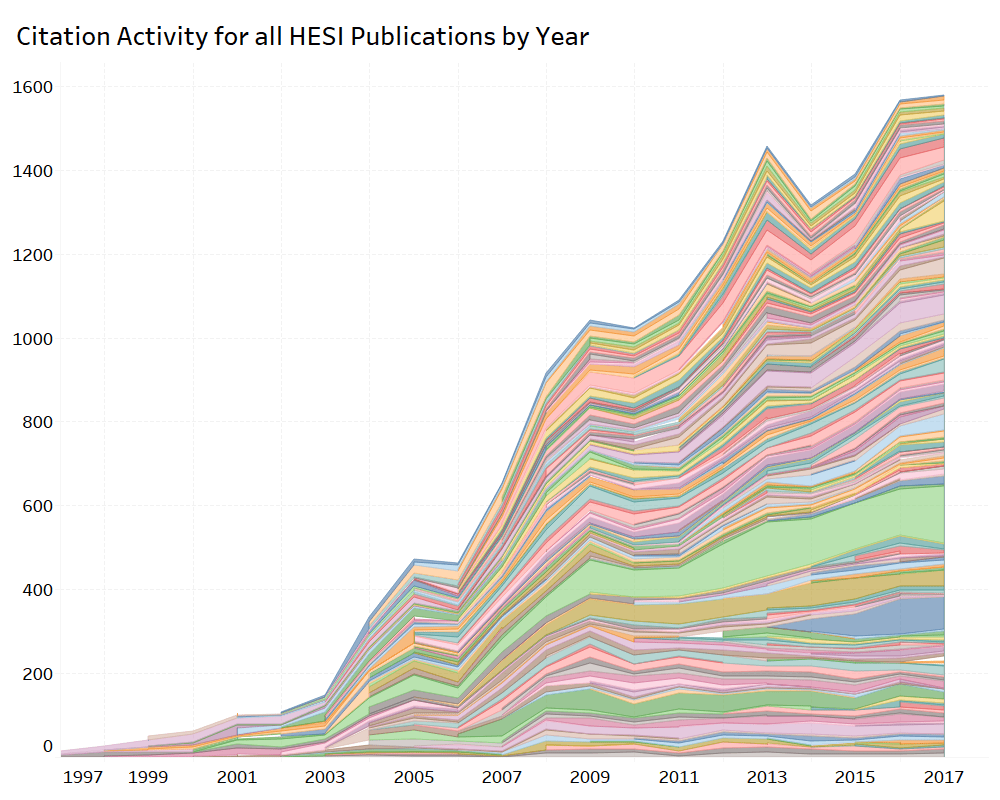
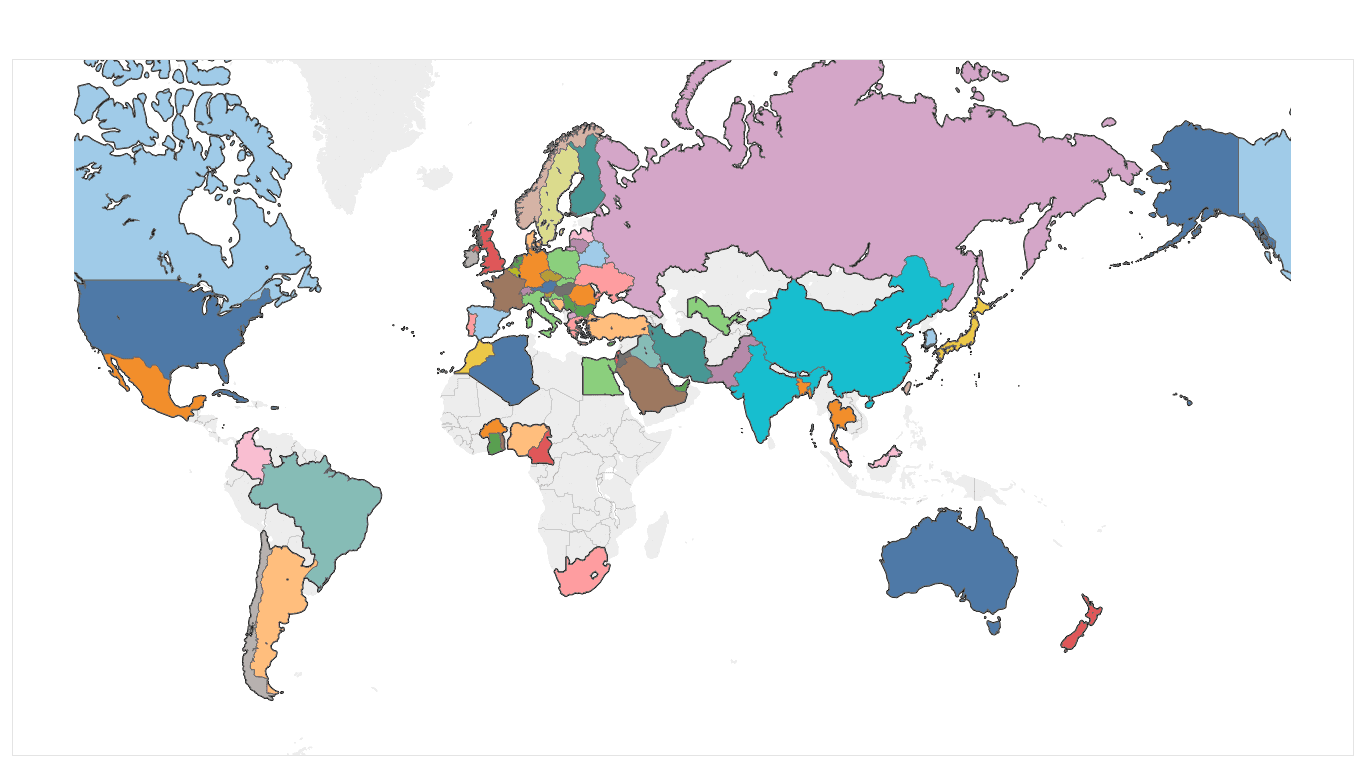
HESI’s scientific programs provide an opportunity for academic, government, and industry scientists to interact on a peer-to-peer basis to address both emerging and long-standing challenges in safety evaluation. HESI conducts a formal citations analysis every 3-5 years, with the most recent citations analysis having been conducted in 2018. The ongoing research, discussions, critiques, and sharing of best practices within HESI are valuable contributions to the practice of science, as evidenced by the examples below.