AOP 296 Published in OECD i-Library

HESI GTTC is proud to report that AOP 296 “Oxidative DNA damage leading to chromosomal aberrations and mutations” is now available on the OECD i-Library! The companion paper (Cho et al., 2022) to this AOP was published last year and given the Editor’s Choice article for the issue. This is the first of a series of genotoxicity AOPs to be published from the committee.
New Health DataWell Lesson Released

HESI and the NSTA have released a new Health DataWell lesson to help high school students advance skills and knowledge needed to become analysts and practitioners of data-driven and equity-focused public and environmental health science. In the Daily Do, “How does where you live effect your risk of heat-related death?”, students evaluate information and consider the limitations of data to investigate why cities have higher heat-wave death rates than suburban areas in the United States.
Explore the free lesson HERE.
Call for Participants!
New Project on Organoids as NAMs for Celiac Disease Modeling
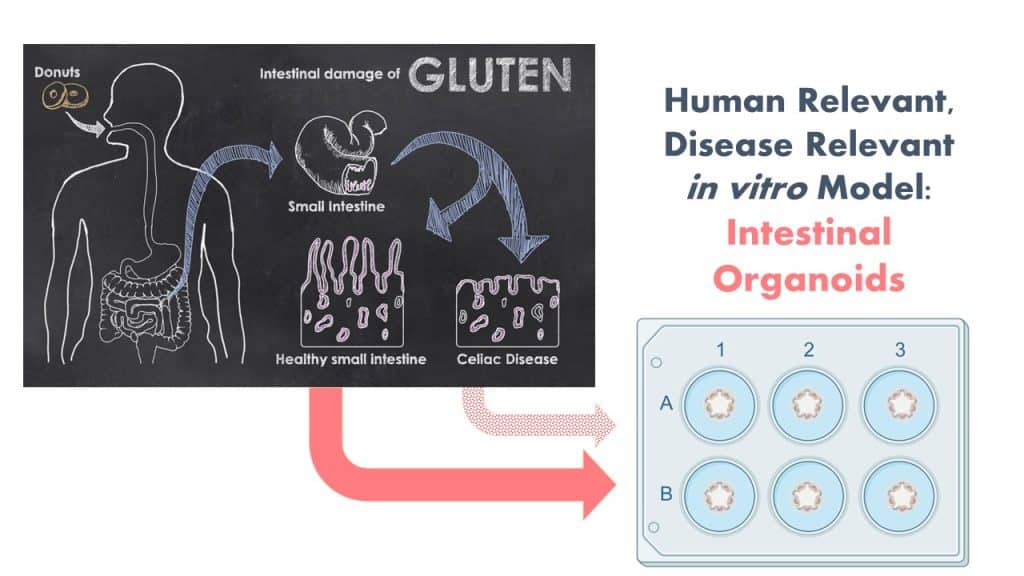
The Protein Allergens, Toxins and Bioinformatics (PATB) Committee is seeking partners to help launch a new project to advance methods for the assessment of gluten-like peptide sequences that could trigger Celiac disease (CD). The proposed pilot study will test the potential of microbial derived peptides to mimic gluten peptides using organoid models.
The project will have relevance for those involved in the evaluation of genetically modified crops, novel foods, or proteins (e.g., for food ingredients) produced via precision fermentation or microbial production systems as well as those with an interest in NAMs for food safety and clinicians/researchers involved in the study of Celiac Disease pathology and immunology.
By involving in vitro NAMs and patient derived materials to generate CD-organoids, this study is anticipated to generate both biological and methodological advancements. We are seeking additional financial support and expertise to ensure that the design and impact of the study is optimized. Your involvement will be enhanced by significant contributions in-kind by a clinical collaborator from Harvard University with expertise in developing these in vitro organoid systems! Peptides will be generated this fall and bench work to launch in early 2024. For more background information, continue reading here.
As with all HESI projects, the success and impact of this initiative will depend on engaging thought leaders and international stakeholders, from government regulators to clinicians, to crop protection, food biotechnology, and biomedical industry experts, academics, and representatives from other relevant organizations.
Interested in finding out more?
Please contact Dr. Lucilia Mouriès at lmouries@hesiglobal.org