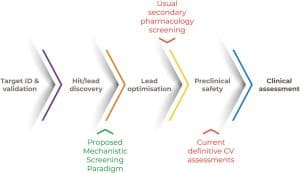
We are thrilled to announce that the Health and Environmental Sciences Institute (HESI) is celebrating a remarkable milestone – 35 years of pioneering research, collaboration, and innovation in the fields of health and environmental sciences!
To commemorate this special occasion, we’ve put together an interactive presentation highlighting some of the most memorable moments and significant achievements from our journey. We invite you all to take a stroll down memory lane and explore the milestones that have shaped HESI into what it is today.
Click here to explore the interactive presentation: HESI 35-Year Timeline
This journey wouldn’t have been possible without the unwavering support of our dedicated members, partners, and stakeholders. Together, we’ve tackled some of the most pressing challenges facing human health and the environment, and we remain committed to driving positive change for the next 35 years and beyond.
Health DataWell Releases New Curriculum for High School Students

Our 8-lesson curriculum unit on Using Data to Understand and Improve Health Outcomes is live on the HESI-NSTA Health DataWell website. Designed for high school (grades 9-12) teachers but great content for many purposes. Please share and let us know what you think!
https://www.nsta.org/health-datawell
This project uses real-world data and case studies to develop students’ understanding of the varied roles of citizens and health professionals in protecting and promoting the health and wellness of their communities.
New HESI Workshop – Cardiac Safety and Drug Development

The HESI Cardiac Safety Committee is hosting a 2-day workshop focused on Cardiac Safety and Drug Development. Virtual and In-person options are available.
Day 1: May 7, 2024; 8:30 am – 5:00 pm ET
Workshop on Validating and Using Cardiac NAMs for Toxicity Screening and Drug Development
Day 2: May 8, 2024; 8:30 am – 5:00 pm ET
Advances in Cardiac Safety Assessments Workshop
Location: Hybrid Virtual Option and In-Person at FDA White Oak, Silver Spring, MD
View the draft agendas and register for free on the event page here.
Contact for more information: Jennifer Pierson (jpierson@hesiglobal.org)
The Health and Environmental Science Institute’s Genetic Toxicology Technical Committee (HESI GTTC) Education and Science Outreach Committee is pleased announce the 2024 awards that will provide opportunities for trainees to attend scientific conferences, workshops, or courses, etc., to build core competencies and transferrable skills, and/or share your research. Awardees will be invited to GTTC’s 2025 Annual Meeting to learn about the emerging projects and science at HESI and to network with experts in the gene toxicology field.
GTTC is pleased to announce TWO awards for 2024:
- Professional Development Travel Award – Provides $1,500 USD for travel to attend scientific conference or workshops.
- Professional Development Training Award (new!) – Provides $4,000 USD for travel specific to training courses, cross-lab trainings, etc. to build core competencies and transferrable skills.
Learn more and download the application here.
2024 DART Professional Development Awards
Apply by 31 May 2024

HESI Developmental and Reproductive Toxicology Technical Committee (DART) is pleased to be able to continue its professional development award in 2024. Two awardees (MS, PhD, Post Doc) will receive $2000 USD towards attending professional meetings or trainings that occurs within the 12-month post application period. The application period is open from April 1 – May 31. Please see the HESI DART website for the 2023 awardees and more information on how to apply.