Announcing the 2024 HESI Board of Trustees: Election Results and New Leadership

We are pleased to announce the results of the 2024 HESI Board of Trustees vote! The below slate has been unanimously elected via electronic ballot and was approved at the June 5th virtual board meeting. Thank you to everyone who participated in the voting process and a big congratulations to our new and returning Trustees!
Current list of the full Board of Trustees is available here.
HESI Board of Trustees 2024 Newly Elected Members (*designates re-nominees):
Public Sector
• Tara Barton-Maclaren, Health Canada
• Roger Chammas, Universidade de São Paulo*
• Roland Froetschl, BfArM Germany
• Yasunari Kanda, National Institute of Health Sciences, Japan
• José Manautou, University of Connecticut*
• Rose Omari, Ghana Science and Technology Policy Research Institute*
• Marc Pallardy, Université Paris-Saclay*
Private Sector
• Patricia Escobar, Merck & Co., Inc*
• Mick Fellows, AstraZeneca
• Sarah Hughes, Shell Health – Americas*
• Robert Landsiedel, BASF
• Gladys Ouedraogo, L’Oréal
• Stefan Pfuhler, Procter & Gamble Company*
A special THANK YOU to our members who rotated of the Board! Your contributions have been invaluable!
 |
 |
 |
 |

 |
 |
TEA Global Challenge

The HESI Committee to Transform the Evaluation of Agrochemicals is pleased to announce the TEA Global Challenge, a competition designed to solicit CREATIVE and CUTTING-EDGE solutions to the modernization of the risk assessment paradigm for crop protection products.
The Objectives of the TEA Global Challenge are to:
- Showcase the feasibility of a new approach for agrochemical safety assessment,
- Encourage knowledge sharing,
- Foster collaboration across disciplines, and sectors.
Anyone is welcome to submit a proposal to solve the problem posed by the TEA Committee. Competitors are encouraged to work in teams, preferable multidisciplinary, although this will not be a selection criterion. Register your team now!
Finalists will be invited to present their case study at the HESI TEA Symposium, in Washington, DC (2025 date TBD), where they will have the opportunity to meet professionals from academia, government and industry with expertise in the field of agrochemicals.
$2000 in travel funds to be used towards a relevant scientific conference (e.g., World Congress on Alternatives and Animal Use in the Life Sciences, SOT) will be awarded to the winners, chosen by the TEA Symposium attendees.
Winners will also be provided support to publish their work if they so desire (pre-publication review by Committee members, contribution to open access fees)
For more information, please, visit the TEA Global Challenge website.
An informational webinar will also be held on June 18 from 10 to 11 am ET. Register HERE to join the webinar.
Shout Out to CT-TRACS!
We are thrilled to announce that the Cell Therapy – Tracking, Circulation, & Safety (CT-TRACS) Committee has reached an incredible milestone: their Tumorigenicity Position Paper (Sato et al., 2019) has now been cited 100 times! This significant achievement highlights the impact and relevance of their work in the scientific community.
Notably, this influential paper was also cited by Nobel Prize winner Shinya Yamanaka, renowned for his groundbreaking development of induced pluripotent stem cells (iPSCs).
Read the full paper here.
Congratulations to the CT-TRACS team for this outstanding accomplishment!


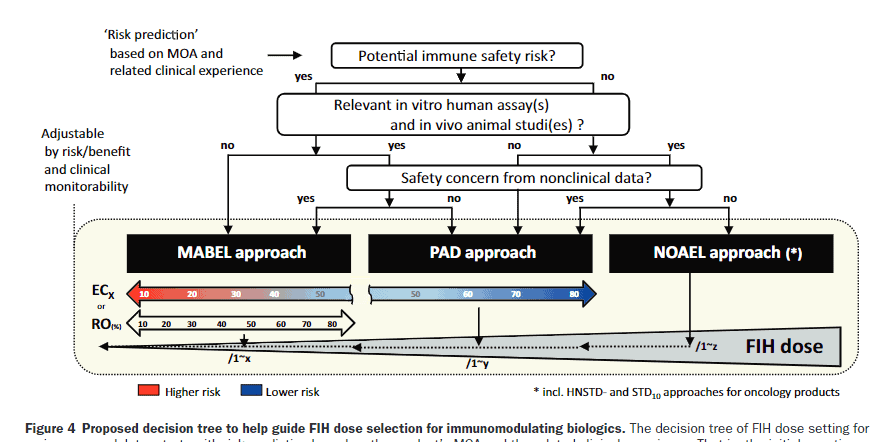

 HESI has teamed up with the US National Science Teaching Association to develop
HESI has teamed up with the US National Science Teaching Association to develop 












