HESI Turns 35!

This year marks HESI’s 35th anniversary. HESI science has been implemented around the world to improve decision making, enhance human health and safety, and preserve the environment. Looking forward to the next 35 years, HESI is excited to continue creating a collaborative environment for scientists from academia, government, industry and NGOs to come together to create science based solutions for a safer more sustainable world.
To learn more about HESI, view our annual reports and monthly newsletters from past years and reach out to get involved!
Genetic Toxicology Technical Committee (GTTC) Nitrosamines Research Program
The HESI Genetic Toxicology Technical Committee is pleased to highlight their Nitrosamines Research Program (NRP). Under the auspices of their Mechanism-Based Genotoxicity Risk Assessment Working Group which aims to demonstrate the usefulness of innovative, mechanism-based testing approaches, the NRP will address knowledge gaps regarding the safety of patients exposed to drugs contaminated with nitrosamine impurities. These impurities are of significant concern since nitrosamines as a class are considered potent mutagenic carcinogens that may increase the risk of cancer if people are exposed to them above acceptable levels.
The NRP has grown since its launch in 2022 and now include four key aims: (1) to develop a protocol for an optimized Ames assay to predict the carcinogenicity of nitrosamines; (2) identify and verify in vitro assays with metabolically competent cells to support nitrosamines risk identification; (3) develop a possible in vivo follow-up strategy to verify Ames data within the frame of ICH M7 and, (4) refine and extend the Carcinogenic Potency Categorization Approach (CPCA) using (Quantitative) Structure–Activity Relationship [(Q)SAR] models and Quantum Mechanics (QM) for more predictive and accurate predictions.
The (Q)SAR-QM working group is just launching, and you can learn more about the proposed aims here.
An overview of the NRP can be found on the Nitrosamines webpage here.
For more information or to get involved please contact Connie Chen and Raechel Puglisi.
COMPARE 2024 is Live!

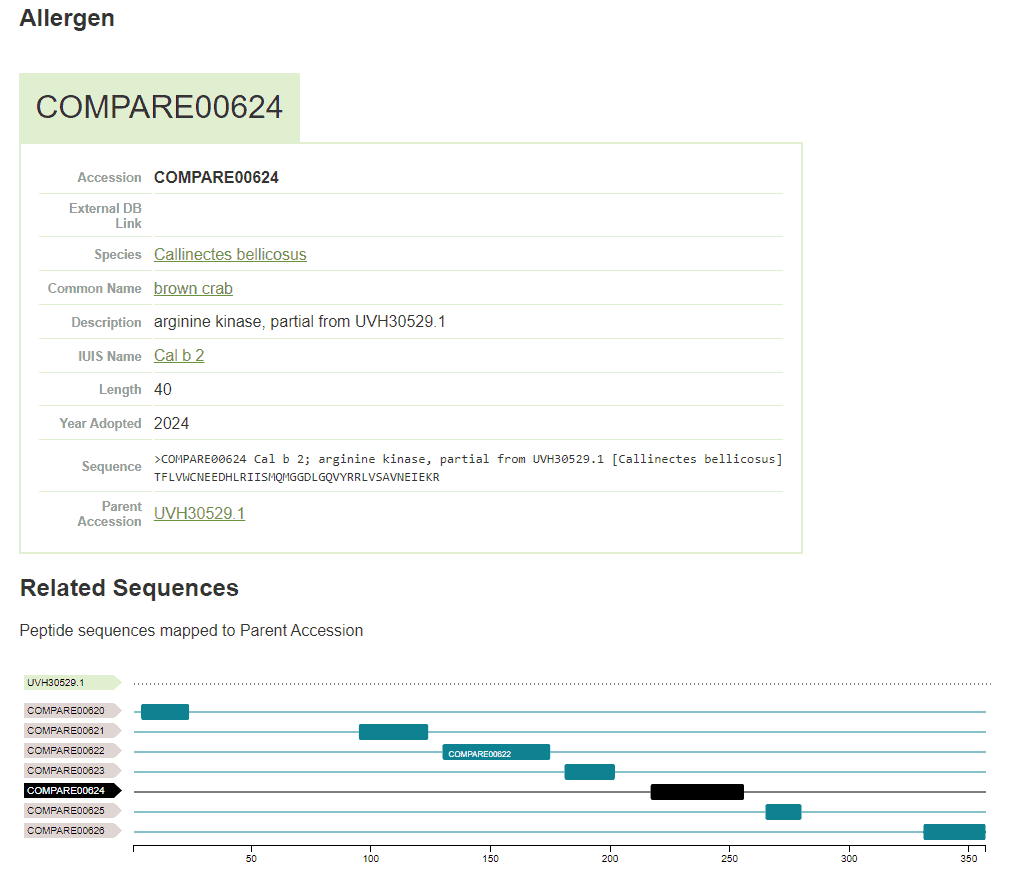 COMPARE 2024, the eighth iteration of the COMprehensive Protein Allergen REsource, is now available! COMPARE is a transparent resource, publicly accessible, for identifying protein sequences that are known allergens, contributing valuable information to the field of allergen research and safety assessment for novel foods. It is being used worldwide, by over 4,500 users across all continents.
COMPARE 2024, the eighth iteration of the COMprehensive Protein Allergen REsource, is now available! COMPARE is a transparent resource, publicly accessible, for identifying protein sequences that are known allergens, contributing valuable information to the field of allergen research and safety assessment for novel foods. It is being used worldwide, by over 4,500 users across all continents.
The new release contains 2,748 entries and a new graphical representation for viewing sequence fragments. It takes 9 months and collaboration with multiple international stakeholders from public and private sector (including end-users; bioinformaticians; molecular allergologists and allergy clinicians) to put together each new version.
Check-it out! https://comparedatabase.org/
Congratulations to the Cardiac Safety Early Career Seminar Series Awardees!

The Cardiac Safety Steering Team is proud to present the 2024 webinar series featuring Early Career Seminar Series Awardees. This competitive award is given to postdoctoral or early career scientists who have compelling research related to cardiovascular safety and risk assessment. Save the dates for this year’s seminars!
Three awardees were selected for the 2024 Seminar Series:
- Dr. Chon Lok Lei, February 22 at 9am ET
- Dr. Victoria Au Yueng, March 22 at 11am ET
- Dr. Alexandra Schaffert, April 5 at 11am ET
Details can be found in the Upcoming Events section below.
HESI Member Scott Auerbach Receives 2024 SOT Arnold J. Lehman Award

Congratulations to Dr. Scott Auerbach of the National Institute of Environmental Health Sciences, National Toxicology Program, on receiving the 2024 Society Of Toxicology (SOT) Arnold J. Lehman Award.
This award recognizes an SOT member who has made a major contribution to risk assessment and/or the regulation of chemical agents, including pharmaceuticals.
Dr. Auerbach has been a HESI member for over a decade. He is involved in the HESI Botanical Safety Consortium Data Analysis Group, the Transforming the Evaluation of Agrochemicals (TEA) Committee and is Chair of the Emerging Systems Toxicology for the Assessment of Risk (eSTAR) Committee and member of the Carcinogenomics, Error Corrected Sequencing and Transcriptomic Point of Departure for Chemical Risk Assessment working groups. Congratulations Scott!


 The
The 












