BE PART OF A NEW HESI PROGRAM ON ADC SAFETY ASSESSMENT!
- Antibody-drug conjugates (ADCs) have the potential to advance patient benefit by providing targeted delivery of therapeutics for both oncology and non-oncology indications.
- Advances in this space will require more robust methods to identify translationally appropriate nonclinical methods for safety assessment of ADCs.
- This newly launching program will leverage HESI’s expertise in academic, government and industry collaboration and study design/execution to define opportunities and guide the selection of new translational methods for the nonclinical safety evaluation of ADCs.
The Health and Environmental Sciences Institute (HESI) seeks your suggestions for priority emerging scientific issues (human or environmental health) that should be addressed through a focused, multi-sector, collaborative program. Proposals will be reviewed in early 2024 and one or more may be selected to form a new scientific program within HESI.
This is NOT a grant program and no direct financial awards to external parties will be made. However, selected programs may receive funding in the form of HESI support for scientific program design, coordination, and staffing. We are seeking topics that focus on the applied human and/or environmental health sciences and that require scientific perspectives and expertise from academia, government, industry, NGOs, clinicians, and/or other research sectors.
Looking for somewhere to start? Learn more about HESI’s existing scientific activities on our committees page and in the 2022 Annual Report. These resources may help guide you when considering your idea, however, HESI welcomes all new proposals whether they are captured in these lists or not.
The deadline to submit proposals for consideration is 15 January 2024.
Click here to learn more about the Emerging Issues process and complete the proposal form!
Cardiac Safety Committee 2024 Early Career Seminar Award Series
Apply by 1 November 2023

The HESI Cardiac Safety Committee seeks Postdoctoral or Early Career researchers working in cardiovascular safety science or a related field for the 2024 Early Career Seminar Award Series. This award offers an opportunity to share your research, learn from and network with experts in the toxicology and safety pharmacology fields from academia, regulatory agencies and pharmaceutical companies.
Award: Selected candidates will receive a $500.00 USD award and be invited to present their research on a public webinar with HESI Cardiac Safety Committee scientists and other invited guests. Webinar dates will be scheduled with the awardees in Q1 2024.
Qualifications and Requirements: Eligible candidates must have a PhD or equivalent degree in biology or related field (cell biology, biochemistry, pathology, biomechanical engineering, or similar); have current post-doc position/appointment or consider themselves an early career scientist (~1-5 years post-academic training) and may not have previously received an award under this program. Open to both domestic and international applicants.
Application: To apply, please send 1 PDF document with the following: 1) completed application form, 2) CV, and 3) one reference letter: to Jennifer Pierson or Claire O’Brien at cardiacsafety@hesiglobal.org.
Deadline for submission: November 1, 2023.
New Project on Organoids as NAMs for Celiac Disease Modeling
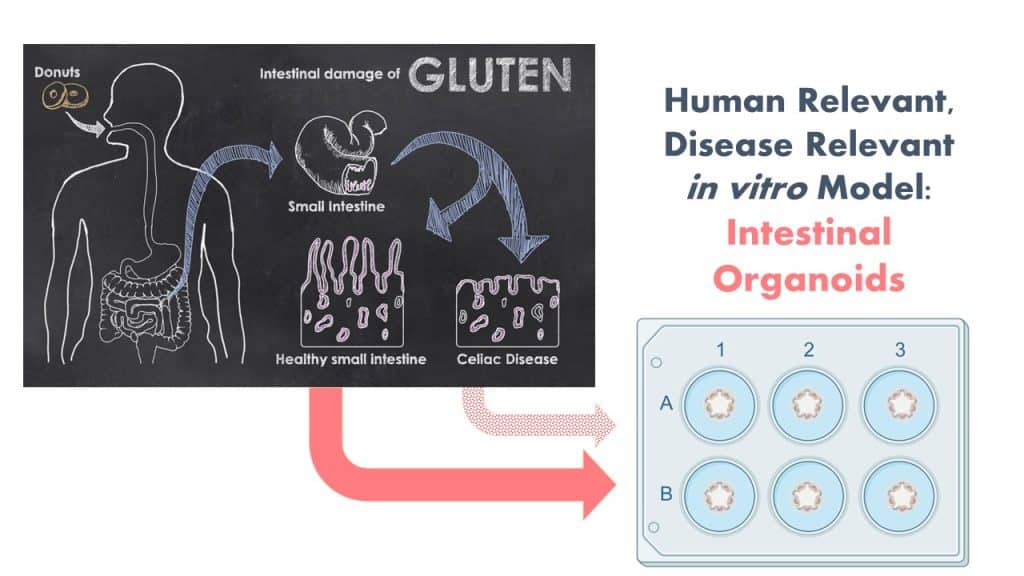
The Protein Allergens, Toxins and Bioinformatics (PATB) Committee is seeking partners to help launch a new project to advance methods for the assessment of gluten-like peptide sequences that could trigger Celiac disease (CD). The proposed pilot study will test the potential of microbial derived peptides to mimic gluten peptides using organoid models.
The project will have relevance for those involved in the evaluation of genetically modified crops, novel foods, or proteins (e.g., for food ingredients) produced via precision fermentation or microbial production systems as well as those with an interest in NAMs for food safety and clinicians/researchers involved in the study of Celiac Disease pathology and immunology.
By involving in vitro NAMs and patient derived materials to generate CD-organoids, this study is anticipated to generate both biological and methodological advancements. We are seeking additional financial support and expertise to ensure that the design and impact of the study is optimized. Your involvement will be enhanced by significant contributions in-kind by a clinical collaborator from Harvard University with expertise in developing these in vitro organoid systems! Peptides will be generated this fall and bench work to launch in early 2024. For more background information, continue reading here.
As with all HESI projects, the success and impact of this initiative will depend on engaging thought leaders and international stakeholders, from government regulators to clinicians, to crop protection, food biotechnology, and biomedical industry experts, academics, and representatives from other relevant organizations.
Interested in finding out more?
Please contact Dr. Lucilia Mouriès at lmouries@hesiglobal.org