SCIENTIFIC PROGRAM MANAGER
Looking for an exciting career in human or environmental toxicology, drug safety, biomedical innovation, regulatory science, epidemiology, exposure-science, and risk assessment — outside of the lab?
Want a great place to grow your career via collaboration with thought-leaders from government, industry, academia, clinic, and NGOs?
Seeking an opportunity to apply your skills in leadership and program management while applying science to real world challenges?
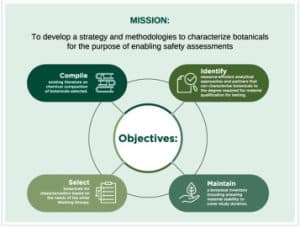
The Health and Environmental Sciences Institute (HESI), a global nonprofit scientific organization, seeks a Scientific Program Manager to help lead collaborative scientific programs in the biomedical and environmental health fields. Successful candidates will have a passion for fostering creative and rigorous scientific collaborations that pool expertise, resources, and technical input from a diverse base of stakeholders. HESI Scientific Managers are responsible for developing, managing, and providing scientific input to large international scientific teams that generate science to protect public health and the environment. HESI offers the opportunity to work with internationally recognized scientists from government, academia, NGOs, clinic, and industry. This responsible position requires leadership skills and the ability to work as part of a team to help realize HESI’s mission of achieving science for a safer, more sustainable world.
This position works in Washington, DC, and reports to the HESI Executive Director.
KEY RESPONSIBILITIES
Provides scientific, strategic, management, and administrative support to collaborative scientific committees involving academic, government, nonprofit, and private sector scientists;
Works with committee members to design and execute novel scientific research programs, publications, workshops, and trainings;
Prepares and monitors committee budgets, assists with communications (technical and non-technical), supports membership development, facilitates workshops and meetings, and contributes to overall HESI strategy and outreach.
QUALIFICATIONS
• A Master’s or Ph.D. in a scientific field such as toxicology, pharmacology, genetics, immunology, epidemiology, genetics, molecular biology, cell biology, pathology, chemistry, environmental health, or related life sciences and a minimum of 3 years of experience in project management, drug or chemical safety evaluation, scientific consulting, and/or regulatory affairs.
• Strong facilitation skills and the ability to work with groups meeting virtually or in person.
• Advanced written and verbal communication skills, outstanding management and organizational ability.
• At least 5 years of experience in project or program coordination.
• Leadership experience and presentation skills.
• Technical/scientific writing.
EXPERIENCE
Expertise in any/all of the following areas preferred:
• Regulatory Science
• Exposure Science
• Drug Safety Assessment
• Chemical Safety Assessment
• Computational Toxicology
• ADME
ABOUT HESI
At HESI, it is our mission to collaboratively identify and help to resolve global health and environmental challenges through the engagement of scientists from academia, government, industry, clinical practice, research institutes and NGOs. We achieve that in a variety of ways:
Create a collaborative environment where scientists from academia, government, industry, and NGOs come together to find solutions that improve health and environmental safety.
Encourage the development of meaningful studies that ask the right questions, structure the right framework, and develop solutions that inform decision-making by both private- and public-sector scientists.
Create a knowledge base that can be easily transferred from the laboratory or journal page to real life.
Individuals interested in applying for this position should send their RESUME with COVER LETTER to Career@hesiglobal.org.
Principals only, please. Unsolicited resumes from third-party agencies will not be considered. Qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, or veteran status.
Learn more about us at www.hesiglobal.org.