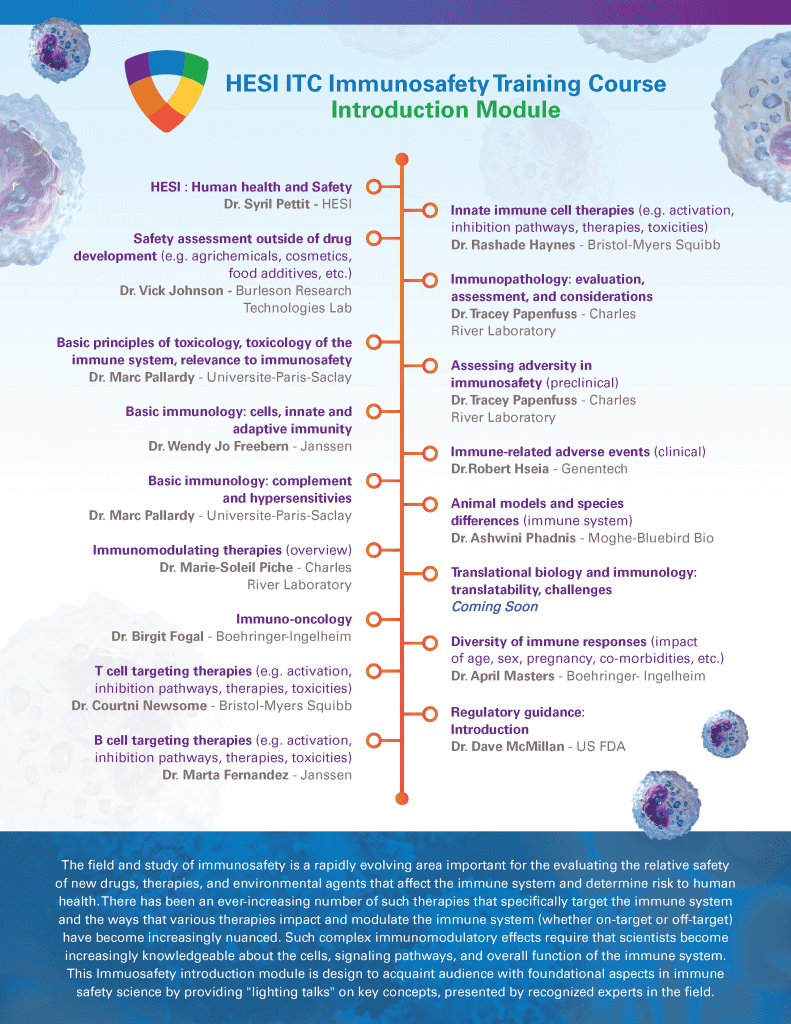
HESI Grants and Government Support Programming

Did you know that HESI has 5 active grants or government funded programs in 2022? As a US-designated nonprofit 501(c)(3) organization, HESI is eligible to apply for and receive funding from a variety of sources to support scientific programs. These current funds are supporting large-scale, multi-site laboratory studies, staff time and scientific programming and many grant-funded programs are also expanded with in-kind work from our committee members. As we are all aware, scientific endeavors are becoming increasingly complex and require more time, effort and resources. HESI’s ability to bring both private and public sector scientists together to collaborate and share resources is one key to success. Interested in learning more about HESI’s grant funded programs? Contact Jennifer Pierson (jpierson@heisglobal.org).
A Gift that Keeps on Giving
BMS partners with HESI THRIVE to make cancer patient’s quality of life a research priority
HESI THRIVE has a received a 25k contribution from Bristol Meyer Squibb’s Grants and Giving Department to support a THRIVE seed grant award in the 2022-2023 cycle. If you and your organization would like to learn more about you can contribute to this initiative, visit the HESI THRIVE page on the HESI website.