
The Nitrosamines research activities exist under the umbrella of the HESI GTTC Mechanism-based Genotoxicity Risk Assessment (MGRA) working group as a “case study” to demonstrate the usefulness of innovative, mechanism-based testing approaches.

GTTC: The mission of the Genetic Toxicology Technical Committee (GTTC) is to improve the scientific basis of the interpretation of results from genetic toxicology tests for purposes of more accurate hazard identification and assessment of human risk; to develop follow-up strategies for determining the relevance of test results to human health; to provide a framework for integration of testing results into a risk-based assessment of the effects of chemical exposures on human health; to promote the integration and use of new techniques and scientific knowledge in the evaluation of genetic toxicology; and to monitor and promote the development of innovative tests and testing strategies.
MGRA working group: The Mechanism-based Genotoxicity Risk Assessment (MGRA) working group aimed to develop a new mechanism-based risk assessment paradigm for genotoxicity that uses a genetic toxicology testing strategy designed from a “clean slate”, incorporating new science and technology to allow for a greater diversity of genomic damage to be addressed, and uses genotoxicity Adverse Outcome Pathways (AOP)s.
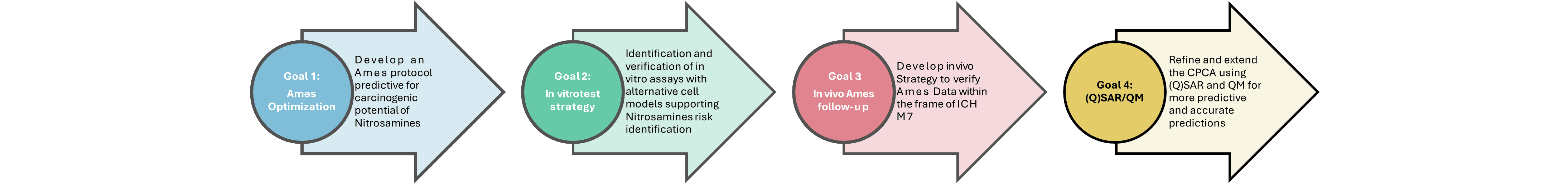
Goal: Develop an optimized Ames protocol that is predictive for the carcinogenic potential of nitrosamines.
Workplan:
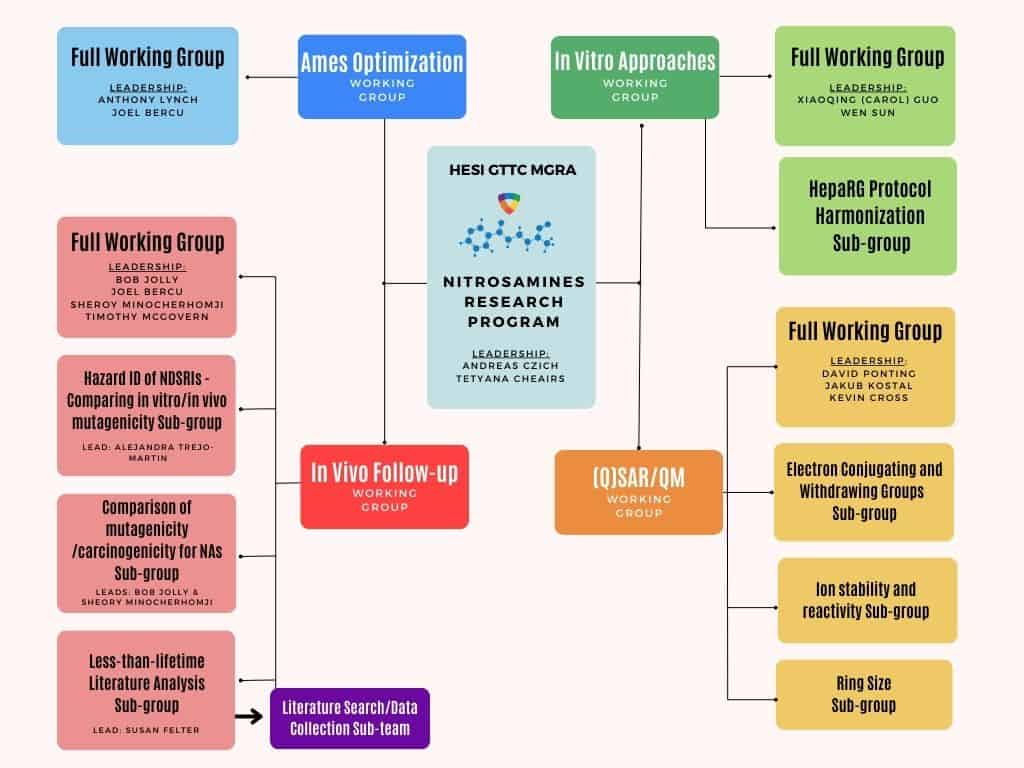
Leadership: Anthony Lynch (GSK), Joel Bercu (Gilead)
Goal: Identification and verification of in vitro assays to assist in nitrosamine risk identification
Workplan: Working group launched in October 2023, with several of the research objectives identified following the FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment on Nitrosamine Impurities in Drugs including:
Leadership: Xiaowen Sun (Pfizer) and Xiaoqing (Carol) Guo (US FDA/NCTR)
Goal: Develop an in vivo strategy to verify Ames data within the frame of ICH M7
Workplan: Working group launched in October 2023, with several research objectives identified following the FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment on Nitrosamine Impurities in Drugs including:
Leadership: Joel Bercu (Gilead), Robert Jolly (Eli Lilly), Sheroy Minocherhomji (Eli Lilly), Timothy McGovern (US FDA)
Goal: Refine and extend the CPCA using (Q)SAR and QM for improved predictive performance.
Working group launched in March 2024, with several research objectives identified following the FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment on Nitrosamine Impurities in Drugs including:
Leadership: David Ponting (Lhasa), Jakub Kostal (George Washington University), Kevin Cross (Instem)
| Government/Regulatory | Academic | Industry | Consulting | |
| ANSM (France) | Colorado State University | AbbVie | Merck | FSTox Consulting |
| BfArM (Germany) | NY Medical College | AstraZeneca | Merck kGA | |
| CBG-MEB (Netherlands) | St. George’s Medical School, University of London | Bayer | MultiCASE | |
| Danish Medicines Agency | Swansea University | BASF | Novartis | |
| EMA | University of Minnesota | Boehringer Ingelheim | PepsiCo | |
| Health Canada | Bristol Myers Squibb | Pfizer | ||
| INRA (France) | Charles River Laboratories | Proctor & Gamble | ||
| NIHS (Japan) | NGO, Non-Profit | Corteva | Roche | |
| RIVM (Netherlands) | American Chemistry Society | Eli Lilly | Sanofi | |
| Swissmedic | Lhasa Limited | Gilead | Takeda | |
| US FDA/CDER | US Pharmacopeia | GSK | Teva Pharmaceuticals | |
| US FDA/NTR | Inotiv | Xenometrix | ||
| Janssen | ||||
| Labcorp | ||||
| Leadscope | ||||
| Litron Laboratories |



Sanofi

New York Medical College
No results.
hesi@hesiglobal.org
Phone: +1-202-659-8404
Fax: +1-202-659-3859
740 15th Street NW, Suite 600
Washington, DC 20005
Sign up for our monthly e-newsletter.