Thymidine Kinase+/− Mammalian Cell Mutagenicity Assays for Assessment of Nanomaterials
- Publication Date :
- Publication Type : Journal Article
- Author(s) : Chen T, Dusinska M and Elespuru R
- Journal Name : Frontiers in Toxicology, Methods and Protocols in Nanotoxicology
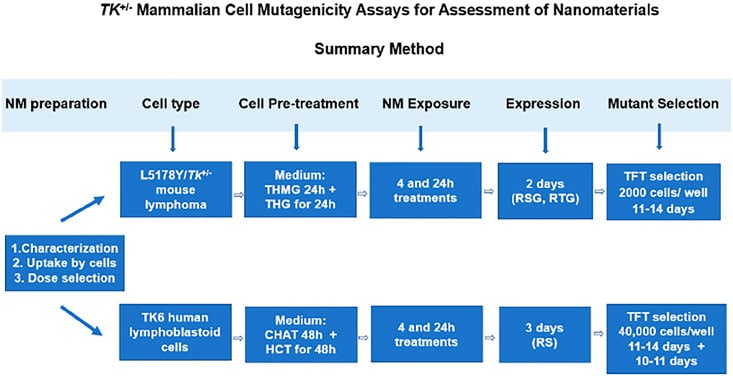 The methods outlined in this article are part of a series of 4 papers designed specifically for genotoxicity assessment of nanomaterials (NM).
The methods outlined in this article are part of a series of 4 papers designed specifically for genotoxicity assessment of nanomaterials (NM).
Read the full paper here:
Thymidine Kinase+/− Mammalian Cell Mutagenicity Assays for Assessment of Nanomaterials. Chen T, Dusinska M and Elespuru R. Frontiers in Toxicology, Methods and Protocols in Nanotoxicology. 8 June 2022. https://doi.org/10.3389/ftox.2022.864753
Other papers in this series are:
- Common considerations for Genotoxicity Assessment of Nanomaterials
- In vivo mammalian alkaline comet assay: method adapted for genotoxicity assessment of nanomaterials
Click to learn more about the Genetic Toxicology Technical Committee