Establishing a Quantitative Framework for Regulatory Interpretation of Genetic Toxicity Dose-Response Data: MOE (Margin of Exposure) Case Study of 48 Compounds with both in vivo Mutagenicity and Carcinogenicity Dose-Response Data
- Publication Date :
- Publication Type : Journal Article
- Author(s) : Nikolai Chepelev, Alexandra S. Long, Marc Beal, Tara Barton-Maclaren, George Johnson, Kerry L. Dearfield, Daniel J. Roberts, Jan van Benthem, Paul White
- Journal Name : Environmental and Molecular Mutagenesis
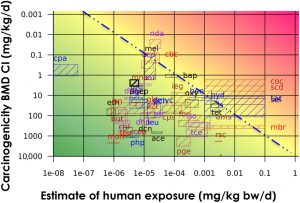 The GTTC’s Quantitative Analysis Workgroup recent publication focuses on the quantitative relationships between carcinogenic potency and mutagenic potency. The group built off the previous benchmark dose (BMD)-based approach by analyzing the carcinogenicity-derived and genotoxicity-derived margin of exposure values (MOEs) from human exposure data of 48 compounds. The findings showed that regulatory decisions based on in vivo genotoxicity dose–response data would be consistent with those based on carcinogenicity dose–response data. Hence in the future, and in the absence of carcinogenicity data, using the in vivo genotoxicity assays could be used to prioritize substances for risk management.
The GTTC’s Quantitative Analysis Workgroup recent publication focuses on the quantitative relationships between carcinogenic potency and mutagenic potency. The group built off the previous benchmark dose (BMD)-based approach by analyzing the carcinogenicity-derived and genotoxicity-derived margin of exposure values (MOEs) from human exposure data of 48 compounds. The findings showed that regulatory decisions based on in vivo genotoxicity dose–response data would be consistent with those based on carcinogenicity dose–response data. Hence in the future, and in the absence of carcinogenicity data, using the in vivo genotoxicity assays could be used to prioritize substances for risk management.
Read full article here:
Establishing a Quantitative Framework for Regulatory Interpretation of Genetic Toxicity Dose-Response Data: MOE (Margin of Exposure) Case Study of 48 Compounds with both in vivo Mutagenicity and Carcinogenicity Dose-Response Data. Chepelev et. al. (2022) Environmental and Molecular Mutagenesis. https://doi.org/10.1002/em.22517
Click to learn more about HESI's Genetic Toxicology Technical Committee