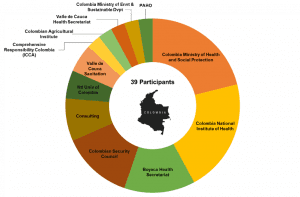
HESI THRIVE is a seed grant program that is innovating translational research and improving quality of life after cancer. By providing researchers with both seed funding and access to critical networks, THRIVE enhances the visibility of the patient need, the value of the research, and the reasons that larger funding entities might elect to incorporate these research streams into future funding priorities.
THRIVE provides seed grants for clinical and translational research and technology-based solutions that enhance our ability to predict when and how adverse effects may occur in patients who have received cancer treatment. The THRIVE grant program is designed to provide seed funding to investigators for the testing of initial hypotheses and collecting of preliminary data to help secure long-term funding by the National Institutes of Health (NIH) and/or other major institutions. This year, we have expanded this opportunity to accept international applications, broadening our reach and heightening the visibility of this important research area. For details on eligibility, funding, and the application process please click here.
THRIVE will accept letters of intent until 10 January 2022. For more information, contact research@hesithrive.org.