Drug hypersensitivity reactions: review of the state of the science for prediction and diagnosis
- Publication Date :
- Publication Type : Journal Article
- Author(s) : Pallardy et al.
- Journal Name : Toxicological Sciences
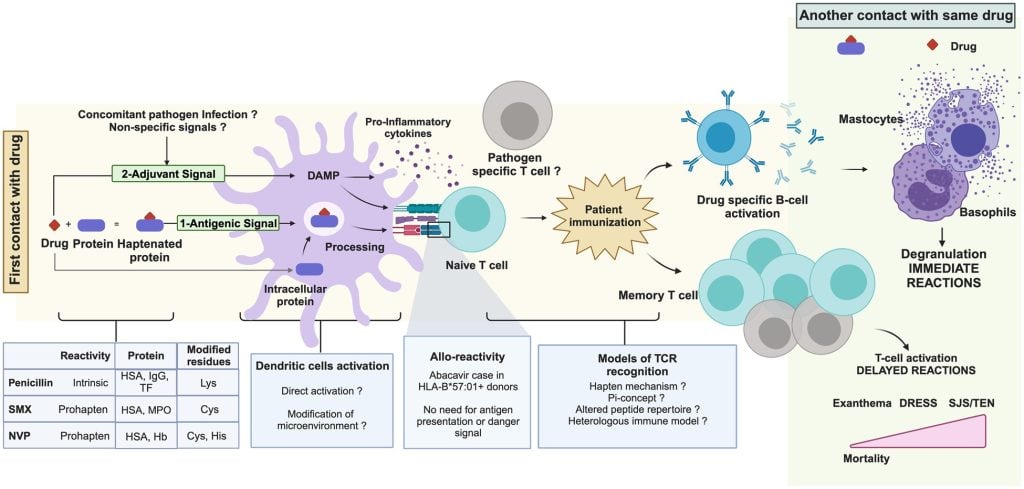
This review from the HESI Immuno-Safety Committee focuses on allergic reactions induced by systemically administered low-molecular weight drugs with an emphasis on drug- and patient-specific factors that could influence the development of drug-induced hypersensitivity reactions (DHRs). Strategies for predicting and diagnosing DHRs, including potential tools based on the current state of the science, are discussed.
Drug hypersensitivity reactions: review of the state of the science for prediction and diagnosis. Pallardy et al. April 2024. Toxicological Sciences. https://doi.org/10.1093/toxsci/kfae046