Beyond MABEL: An Integrative Approach to First in Human Dose Selection of Immunomodulators by the Health and Environmental Sciences Institute (HESI) Immuno-Safety Technical Committee (ITC)
- Publication Date :
- Publication Type : Journal Article
- Author(s) : Mineo Matsumoto, Joseph Ryan Polli, Suresh K. Swaminathan, Kaushik Datta, Cris Kampershroer, Marie C. Fortin, Smita Salian-Mehta, Rutwij Dave, Zheng Yang, Payal Arora, Masanori Hiura, Mizuho Suzuki, Frank R. Brennan and Jean Sathish
- Journal Name : Clinical Pharmacology & Therapeutics
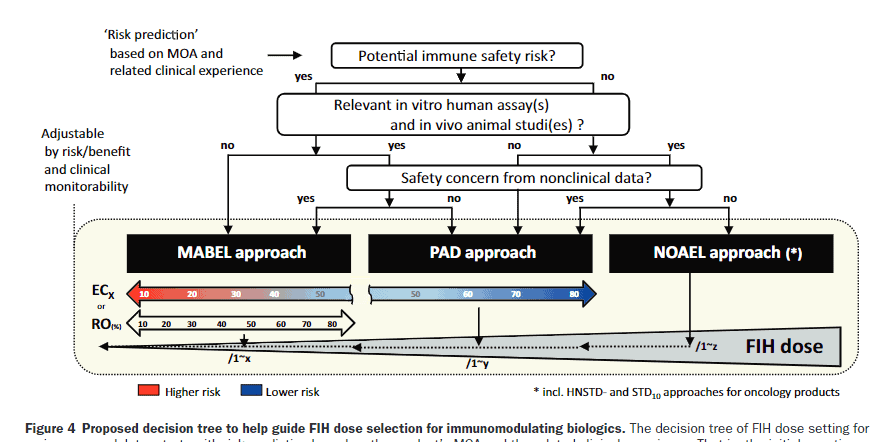
We are thrilled to announce the publication of a groundbreaking white paper by the HESI Immuno-Safety Technical Committee, now available in Clinical Pharmacology & Therapeutics. This pivotal work delves into the integrative approach to first-in-human dose selection of immunomodulators, shedding light on the critical challenges and strategies involved in determining the appropriate dosage in clinical trials.
Congratulations to all the authors for their exceptional contributions!
A special shout-out to Mineo Matsumoto and Joseph Ryan Polli for their years of dedication and persistence in bringing this publication to life. Your hard work and commitment have been instrumental in advancing our understanding in this crucial area of clinical pharmacology.
Check out the full paper here: Beyond MABEL: An Integrative Approach to First in Human Dose Selection of Immunomodulators by the Health and Environmental Sciences Institute (HESI) Immuno-Safety Technical Committee (ITC). Matsumoto and Polli et. al., June 2024. Clinical Pharmacology & Therapeutics. https://doi.org/10.1002/cpt.3316