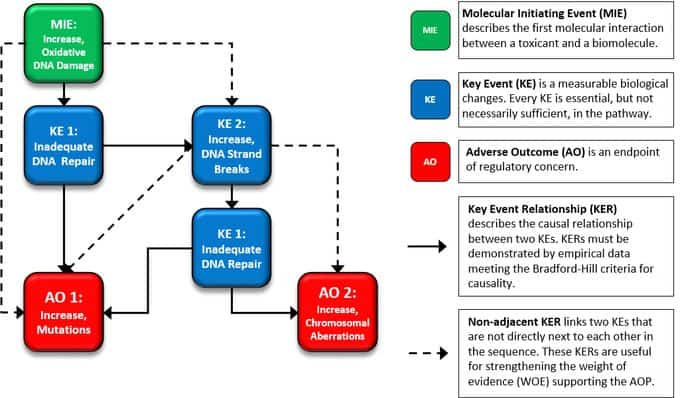
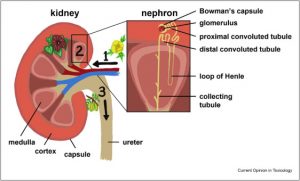
The Health and Environmental Sciences Institute (HESI) & Safety Pharmacology Society (SPS) present a new, on-demand training opportunity: A closer look at the new ICH E14/S7b Q&A’s and Training Materials. The target audience includes all sponsors (small and large; other stakeholders in pharma), safety pharmacologists and toxicologists participating in the delivery of the nonclinical data, clinicians, and regulators. See the course content below.
Overview of the ICH E14/7B Q&A Training Materials
Introduction – David Strauss, FDA, United States
Integrated Risk Assessment – Zhihua Li, FDA, United States
In Vitro – Derek Leishman, Lilly
In Vivo – Hugo Vargas, Amgen
Conclusions – David Strauss, FDA, United States
Case Studies and Scenarios for 5.1
Moderator: Christine Garnett, FDA, United States
Speakers – Corina Dota, EFPIA & Wendy Wu, FDA, United States
Topics covered: E14 Pathways and New Options, Clinical Scenarios, Integrated Nonclinical Data
(in vitro & in vivo)
Case Studies and Scenarios for 6.1
Moderator: Christine Garnett, FDA, United States
Speakers – Hugo Vargas, Amgen & Flora Musuamba Tshinanu, FAGG-AFMPS, Europe
Topics covered: E14 Pathways and New Options, Clinical Scenarios, Integrated Nonclinical Data
(in vitro & in vivo)
Summary of integrated risk data
Training Goals
1. Recognize when nonclinical data may be used in the regulatory QT assessment, including understanding the difference between 5.1 and 6.1.
2. Recognize quality hERG and in vivo studies:
a. Are the studies of reasonable quality with the appropriate quality measures?
b. Is the study consistent with the performing lab’s experience with reference agents?
3. Gain familiarity with clinical exposure definitions and how margins are defined to understand the hERG and in vivo margin.
4. Recognize a double-negative nonclinical package.
5. Understand what information is needed to justify the integrated QTc risk assessment.
6. Understand that there can be mitigation around some of the nonclinical study features.
7. Understand timing of various assays and how to progress to clinic.
8. Understand the flexibility of these guidelines and what alternatives may be implemented.
This training is being provided complimentary for SPS and HESI members, and offered to nonmembers for $49.00 USD until 31 December 2022.
Click to link to training course
Contact Jennifer Pierson at jpierson@hesiglobal.org for details.