New Visualization Feature in COMPARE Database Bioinformatics Tool
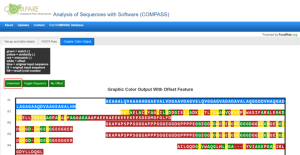
The COMPASS (COMPare Analysis of Sequences with Software) tool in HESI’s COMPARE Database now has an added feature that enables users to analyze comparisons of FASTA sequences using a color-coded output, in addition to the existing table view. This new visual display allows users to quickly identify regions within a query sequence that have similarities with one or more of the 2,248 allergen sequences contained within the COMPARE Database. The display also allows visualization of multiple alignments in one graphic output. Visit COMPARE at comparedatabase.org and select “Run COMPASS” to explore the new feature, and learn about other tool updates under the “Updates” tab.
Survey Open: DART QSAR Modeling of Rodent Placental Transfer Project
The DART Committee’s QSAR Modeling of Rodent Placental Transfer project is looking to collect data on maternal-fetal exposure along with physiochemical descriptors on a variety of small molecules to improve a predictive model for fetal drug exposure in the rat. Integrating exposure data with other alternative assays that predict teratogenicity may broaden our understanding of the effects observed in embryo-fetal development (EFD) studies and strengthen our interpretations by bridging in vitro and in vivo outcomes. If you have maternal-fetal exposure data that you can contribute or you are interested in participating in the study, please fill out this brief survey to confirm your interest and to learn more about the project.
UVCB Publication in Environmental Toxicology and Chemistry

Substances of unknown or variable composition, complex reaction products, or biological materials (UVCBs) pose unique risk assessment challenges to regulators and product registrants. These substances can contain many constituents, sometimes partially unknown and/or variable, depending on fluctuations in their source material and/or manufacturing process. Based on the output of a 2016 UVCB Committee workshop, this publication examines current practices for UVCB risk assessment and reveals a need for a multi‐pronged and transparent approach integrating whole‐substance and constituent‐based information. Continued collaboration of stakeholders representing government, industry, and academia will facilitate the development of practical testing strategies and guidelines for addressing regulatory requirements for UVCBs. Read the full publication here.
Welcome to New HESI Staff!
From left to right: E’Lissa Flores, PhD, and Raechel Puglisi, MPH
Please join us in welcoming our newest staff members, E’Lissa Flores, PhD, and Raechel Puglisi, MPH! E’Lissa joined HESI earlier this month as a Scientific Program Manager and will be co-managing several HESI projects, including the Genetic Toxicology Technical Committee (GTTC), Immuno-Safety Technical Committee (ITC), and the Cardiac Safety Committee. Prior to joining HESI, E’Lissa worked at the Milken Institute’s Center for Strategic Philanthropy where she advised philanthropists and nonprofit foundations to help manage several neurology-related disease projects, including overseeing a $12 million grant program and developing a novel fellowship award. E’Lissa received her Bachelors of Science in Biology from Stony Brook University and her doctoral degree in Translational Biomedical Science from the University of Rochester School of Medicine and Dentistry.
Raechel is our new Scientific Program Associate and has joined the team full-time after interning with HESI in 2019. Raechel received her BA in Human Biology from the University of Kansas and her MPH with a focus in Environmental Health Science and Policy from the George Washington University. In her new role as Scientific Program Associate, she supports staff on a broad range of HESI programs, with most of her work focused on RISK21 projects.
COMPARE Database Presentation at Chinese Society of Allergy 2020 Meeting

The HESI COMPARE Allergen Database was represented at the Chinese Society of Allergy 2020 Meeting on 11-13 September 2020. COMPARE Database Steering Team Member, Prof. Gao Zhongshan (Zhejiang University, China), presented during the “Translational Allergy Session” on 12 September 2020. For more information on the COMPARE Allergen Database, please visit comparedatabase.org or contact program managers Dr. Lucilia Mouriès (lmouries@hesiglobal.org) and Dr. Shermaine Mitchell-Ryan (smitchell-ryan@hesiglobal.org).
PBPK Symposium: Opportunities and Challenges in Using the Kinetically Derived Maximum Dose Concept to Refine Risk Assessment
The HESI PBPK Committee is co-sponsoring a symposium on 30 September 2020 with the U.S. Environmental Protection Agency (EPA) and the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICETAM) on “Opportunities and Challenges in Using the Kinetically Derived Maximum Dose Concept to Refine Risk Assessment.” Presenters will describe the incorporation of pharmacokinetics into the design of in vivo toxicity testing studies and discuss how the kinetically derived maximum dose can be useful in dose selection and other aspects of study design. The half-day symposium is open to the public and will be recorded. Click here to register.
For more information, please contact Dr. Michelle Embry (membry@hesiglobal.org).
eSTAR Committee and
AAPS Webinar Series

The HESI eSTAR Committee has co-organized a webinar series with the American Association of Pharmaceutical Scientists (AAPS). These webinars are free and open to the public, so please feel free to share the links! Special thanks to Dr. Shraddha Thakkur (U.S. FDA) for helping to build this connection and the AAPS staff for providing the webinar hosting.
Webinar 1: 24 September 2020 at 12:30 PM EST
Development of Next-Generation Biomarkers for Detecting Kidney Toxicity
Dr. Alison Harrill (National Institute of Environmental Health Sciences)
Register here
Webinar 2: 8 October 2020 at 12:30 PM EST
Aligning Regulatory Agencies and the Pharmaceutical Industry Toward Reducing Toxicity Associated Drug Development Costs, Timelines, and Attrition: Two Year Rodent Carcinogenicity Testing
Dr. Frank Sistare (Merck)
Register here
Webinar 3: 15 October 2020 at 12:30 PM EST
Development and Regulatory Approval for Toxicogenomics (TGX) Biomarker to Detect DNA Damage-Inducing Agents
Dr. Carole Yauk (University of Ottawa)
Register here
For more information, please contact Dr. Syril Pettit (spettit@hesiglobal.org).
Save the Date! PATB Committee Protein Toxins Workshop
The HESI Protein Allergens, Toxins, and Bioinformatics (PATB) Committee is co-hosting a virtual workshop in collaboration with the Society of Toxicology (SOT) Food Safety Specialty Section titled “From Protein Toxins to Applied Toxicological Testing” in the context of safety assessment of novel foods and feeds. The virtual workshop will take place on 21–22 October 2020 from 9:00 AM to 1:00 PM EST each day and is open to all interested participants. It will cover the state of the science in protein toxins biology (structure, activity, MOA, etc.), current bioinformatics approaches used to identify and characterize protein toxins, with the ultimate goal to leverage computational biology and in silico approaches for determining the potential of a protein to present toxic properties.
The Protein Toxins Workshop will serve the dual purpose of learning about the advances in protein toxin research presented by recognized international academic experts in the field and assessing the translation of knowledge to practical applications (e.g., into weight-of-evidence approaches for the safety assessment of biotechnology products; relevance, applicability, and limitations of existing protein toxin databases and tools for risk assessment). The desired outcome of this event is to help inform the development of contemporary and scientifically robust approaches for the identification and classification of potential toxicity risk in novel proteins.
For more information, please contact Dr. Lucilia Mouriès (lmouries@hesiglobal.org).
From the Leadership

September 2020 is unlike any other as a result of the continued global COVID-19 pandemic. Despite this challenge, we are fortunate that the HESI organization and its programs are continuing actively and impactfully. For the last 30+ years, HESI has operated with a “virtual teams” model that engages scientists from different locations around the globe. As a result, our ability to move efficiently into a fully remote work environment has required only minimal adjustments. We continue to meet routinely with our committee members, Board of Trustees, and colleagues via phone and web-conferencing, and, as a result, projects are progressing well. Although most of our laboratory based initiatives were slowed in the first half of the year, many are resuming activities now. Our planned in-person meetings for 2020 have all been convened online. While we miss the informal networking of a live event, the upside is that many of our meetings have seen a significant increase in participation because there are no associated travel costs or travel time.
While we all look forward to calmer times ahead, we are proud of our ability to continue to develop timely and relevant science even within this turbulent environment. Thanks to all who have continued to support and engage in HESI’s work. Mask Up, Science On.
Syril D. Pettit, DrPH, MEM
HESI Executive Director


