One of HESI’s core strengths is in its convening power – the ability to draw together scientists from around the globe representing various sectors to help resolve global health and environmental challenges. HESI’s scientific portfolio reflects this strength, with 16 scientific committees and two HESI-led consortia (you can read all about them on the HESI website!) comprising over 1,000 participants. I wanted to take some time in this edition of Insights to highlight two relatively new HESI initiatives that have taken convening to a new level. In addition to the traditional multi-sector stakeholder involvement that underpins all HESI programs, these projects have created connections between existing programs, working to leverage committee expertise and knowledge to connect the dots and amplify their specific project goals.
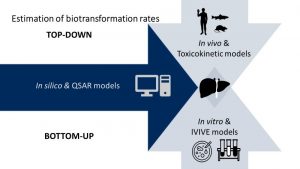
The Botanical Safety Consortium (BSC) was initiated in March 2020 with the aim of evaluating the suitability of in silico and in vitro approaches to assess the safety of botanical dietary supplements. The broad scope of the BSC, which covers various toxicity endpoints, has led to fruitful collaborations and information sharing with the DART, GTTC, Cardiac Safety, and PBPK Committees, while additional partnerships are anticipated to evolve as the BSC laboratory work gets underway.
The Transforming the Evaluation of Agrochemicals (TEA) Committee began work in 2021 and has a wonderful “project landscape” map on the HESI website – check it out! Although the project is focused on the development of fit-for-purpose safety evaluation strategies for agrochemicals, the challenges and opportunities in the map could be applied to the majority of the programs in HESI’s portfolio. Recognizing these connections, the project was specifically designed as an integrative program within HESI and elsewhere, and as a result, has cited the need and opportunity to collaborate and amplify the work-products of nine different programs (and counting) within the HESI portfolio.
It is wonderful to see new initiatives taking shape that are using the amazing expertise that our committee members, project participants, and staff provide, and continuing to develop these integrative, inter-connected programs.
For more information about either of these programs, please contact me (membry@hesiglobal.org), Sandrine Deglin (sdeglin@hesiglobal.org – TEA Committee), or Connie Mitchell (cmitchell@hesiglobal.org – BSC).
Michelle Embry, PhD
HESI Associate Director, Environmental Science