HESI Animal Alternatives Paper One of
Environmental Toxicology and Chemistry’s 2019 Top Downloads
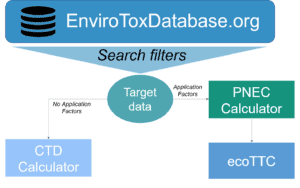
The HESI Animal Alternatives in Environmental Risk Assessment Committee’s paper “Creation of a Curated Aquatic Toxicology Database: EnviroTox” (Connors et al. 2019) was one of Environmental Toxicology and Chemistry’s most downloaded papers in 2019. The EnviroTox Database is a public database of ecotoxicological data which brings together more than 91,000 curated records for more than 4,000 chemicals across 1,500 species. Learn more about the Animal Alternatives in Environmental Risk Assessment Committee here.
HESI Board of Trustees Meeting &
2020 Initiatives

On 7-8 January, the HESI Board of Trustees met to review 2019 achievements and challenges, approve a 2020 organizational budget, progress the strategic plan, and refine Board priorities for 2020. The Board recognized significant new progress in 2019 with respect to achieving its self-identified goal of diversifying HESI’s funding model. As of 2020, the funding committed for HESI’s scientific programs will be approximately 40% public sector and 60% private sector. Continued organizational growth in public and private sector membership was also highlighted. The pending (February 2020) launch of HESI’s first ever publicly initiated and funded consortium (the Botanical Safety Consortium as founded via a Memorandum of Understanding and funding commitment from NIEHS, FDA, and HESI) was recognized as an important milestone in organizational history. Integration and citation of HESI science by national and international safety assessment and public health entities was cited as a critical and ongoing contribution of the programs. Updates from each of HESI’s 16 scientific committees were presented at the meeting by the program’s scientific staff. After considering the portfolio in its entirety, a Board vote was taken to signal their endorsement for the portfolio’s depth, breadth, and rigor.
The Board developed initial plans to a) build HESI’s scientific presence and health impact in global regions with developing economies and b) enhance HESI’s visibility and engagement of academic scientists. Further details on these evolving efforts (and opportunities to help support them) are available upon request.
2020 HESI THRIVE Award Announcement

THRIVE is a competitive seed grant program designed to support research that helps to predict, reduce, or prevent adverse events associated with life-saving cancer therapy. We are pleased to announce this year’s grant award winners, selected from nearly 80 proposals received from more than 35 different institutions. Each of the winning proposals, listed below, will receive $50,000 in funding to support their research in making cancer patient quality of life an active research priority.
2020 HESI THRIVE Grant Award Winners
HESI THRIVE Research Study: Stop LCNP – High dose steroid therapy for late radiation-associated lower cranial neuropathy
- Katherine Hutcheson, PhD, Stephen Y. Lai, MD, PhD, and Karin Woodman, MD
- The University of Texas, MD Anderson Cancer Center
- This clinical project seeks to use high dose steroid therapy to improve swallowing and speech function in long-term survivors of head and neck cancer (HNC) who have nerve dysfunction as a late side effect of their cancer treatment.
HESI THRIVE Research Study: FMT treatment for immune checkpoint inhibitor–mediated colitis
- Yinghong Wang, MD, PhD, MSc
- The University of Texas, MD Anderson Cancer Center
- This prospective study seeks to use fecal transplantation to reduce immune-mediated colitis (which is currently extremely difficult to treat) in patients with melanoma and genitourinary cancer who are receiving immunosuppressive therapies.
HESI THRIVE Research Study: Targeting complement signaling to ameliorate cranial radiation-induced brain injury
- Munjal M. Acharya, PhD, Andrea J. Tenner, PhD
- University of California, Irvine
- This study seeks to improve long-term outcomes for brain cancer survivors by using a genetically modified mouse model to explore the biological mechanisms associated with chronic, radiation-induced cognitive damage.
HESI THRIVE Research Study: Utilization of a pharmacogenomics approach to decrease incidence of vincristine-associated peripheral neuropathy in pediatric oncology patients
- Elizabeth Sokol, MD, and Angela Waanders, MD
- Ann & Robert H. Lurie Children’s Hospital of Chicago
- This study seeks to genetically stratify (and ultimately protect) pediatric cancer patients who may experience enhanced toxicity from a commonly used chemotherapeutic agent – vincristine.
By providing researchers with both seed funding and access to critical networks, THRIVE will enhance the visibility of the patient need, the value of the research, and the reasons that larger funding entities might elect to incorporate these research streams into future funding priorities. To help support THRIVE or learn more, please visit our website here.
CT-TRACS Committee in
Tokyo and Paris
The HESI CT-TRACS Committee will be represented at the upcoming IABS Cell Therapy Conference on 4-5 February 2020 in Tokyo, Japan. HESI was invited to speak and co-chair Session 3, where the CT-TRACS Tumorigenicity Multi-Site Study initiative “Tumorigenicity Assessment of Cell Therapy Products” will be presented and discussed. For the full meeting program, click here.
Additionally, the CT-TRACS Committee will be presenting a 90 minute session titled “Imaging Cellular Therapeutics: HESI CT-TRACS Joint Session” at the International Society of Cell & Gene Therapy (ISCT) Meeting which will be held in Paris, France on 27-30 May 2020. Guest speakers will provide insights into how non-invasive in vivo cell tracking technologies and methods can provide unique opportunities to optimize efficacy of cell-based therapies and aid in the assessment and management of eventual toxicities, and ultimately, benefit their clinical translation. Stay tuned for the confirmed time slot and find additional meeting details here.
For questions, contact Lucilia Mouriès (lmouries@hesiglobal.org).
FDA/CDER – HESI
Immunomodulators and Pregnancy Risk Assessment Workshop

FDA/CDER and HESI will be hosting a workshop in Silver Spring, Maryland on 11 February 2020 to discuss both current and novel methodologies in preclinical and translational safety assessment of pregnancy risk associated with immunomodulatory therapy. Through the sharing of case examples, followed by a longer in-depth discussion within each session, the goal is to begin to address gaps in biology, current tools, and other aspects of pregnancy risk that need to be considered during drug development. Learn more and register here. Registration will close on 5 February 2020. Due to the format and structure of this workshop, attendance is restricted to in-person attendees only.
RISK21 Committee
Risk Assessment Summit

The HESI RISK21 Committee is hosting a Risk Assessment Summit on 18-19 February 2020 in Washington, DC. This event will provide a platform to a broad group of stakeholders to discuss scientific, transparent, and efficient approaches to risk assessment and to identify risk assessment challenges. It will also create an opportunity to share risk assessment case studies illustrating the key concepts and principles underpinning the RISK21 framework and its applications. Learn more and register here. Feel free to contact Sandrine Deglin (sdeglin@hesiglobal.org) or Michelle Embry (membry@hesiglobal.org) with any questions you may have.
Cancer Immunotherapies
Training Course

The HESI Immuno-Safety Technical Committee (ITC) is holding a training course in Tres Cantos, Spain, on 22–23 April 2020 on the topic of cancer immunotherapies. This two-day course will provide the foundation biology, describe therapeutic approaches across the various modalities, and discuss other safety-related issues. For more information and to register, please visit the website or contact Stan Parish (sparish@hesiglobal.org).
From the Leadership

Happy 2020! We had a vigorous start to the year with a HESI Board meeting just seven days into the new decade. As described above, this meeting provided the opportunity to highlight 2019 progress with regard to diversifying HESI’s funding model, adopting new scientific programs, and broadening our stakeholder base. That said, we recognize the time and resource pressures that all of our stakeholders face and are committed to continuing to innovate our approaches to maximize impact and enhance organizational efficiencies. This year, we are also poised to explore growth in geographic regions where there are disproportionate health and environmental burdens, but historically relatively little HESI activity (e.g., African continent). If you would like to be a part of these new efforts – please contact cnobles@hesiglobal.org to get engaged. Looking forward to another a year of science for a safer, more sustainable world.
Syril D. Pettit, DrPH, MEM
HESI Executive Director

