Cardiac safety remains a serious issue during drug development. The mission of HESI’s Cardiac Safety Committee is to improve public health by reducing unanticipated cardiovascular-related adverse effects from drugs or chemicals, and to develop innovative approaches to support early detection and prediction as well as improved understanding of cardiovascular toxicology and pathobiology. This publication in Toxicological Sciences by the committees’ Pro-Arrhythmia Working Group shows that nonclinical models used to predict cardiac safety during drug development do not absolutely replicate the clinical conditions; however, best practices can be used to improve the nonclinical assay quality and achieve the highest predictive power.
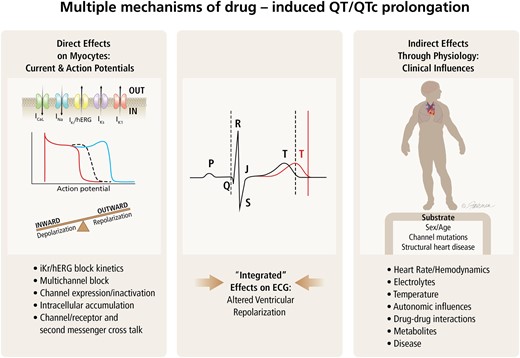
The QT interval is the section on the electrocardiogram (ECG) that represents the time it takes for the electrical system to fire an impulse through the ventricles and then recharge. It is translated to the time it takes for the heart muscle to contract and then recover. Evaluating whether a new medication prolongs the QT interval, which is a risk factor for ventricular arrhythmia, is critical for safe drug development and is evaluated using both in vivo and in vitro models during nonclinical drug development. The relevance of the QT interval as a sole indicator of risk is questionable because many studies confirm that QT prolongation alone does not necessarily precede the development of arrhythmias. Since drug safety scientists must act in a cautious manner, the development of a drug is likely to be discontinued if there are nonclinical indicators of QT prolongation, no matter how extensive the validation studies.
A 3-phased project was conducted by the HESI Pro-Arrhythmia Working Group starting with a detailed literature review and followed by a collaborative HESI-FDA database of 150 new drug candidates to evaluate how predictive nonclinical studies are to clinical outcomes. Phase three included a closer examination of several drugs from the database to identify possible reasons for the lack of predictivity from nonclinical to clinical outcomes. Results from the literature review and database show that nonclinical models do not absolutely replicate the clinical conditions they are used to predict; however, best practices can be used to improve the nonclinical assay quality and achieve the highest predictive power. (1) Assays must cover a broad and appropriate range of drug concentrations or doses to include, if not exceed, estimated clinical exposures, (2) standardized study conditions should be met, and (3) additional drug information, such as plasma protein binding properties, should be used to complement data analysis and interpretation. Application of the recommended best practices outlined in the article should enhance the overall quality of data and improve the interpretation and translation of nonclinical to clinical data. Although gaps exist in the translation of nonclinical assays to the clinic, we hope the content of this article allows researchers and clinicians to have increased confidence in those preclinical tests and encourages them to develop novel assay platforms and improve current methodologies. By understanding how predictive the preclinical studies are to the clinical outcomes, we can then take a closer look at why some drugs are not predicted preclinically and how to improve on that.
Click to read the full article:
The Challenges of Predicting Drug-Induced QTc Prolongation in Humans. Jean-Pierre Valentin, Peter Hoffmann, Catherine Ortemann-Renon, John Koerner, Jennifer Pierson, Gary Gintant, James Willard, Christine Garnett, Matthew Skinner, Hugo M Vargas, Todd Wisialowski, Michael K Pugsley. Toxicological Sciences. 11 February 2022
Click to learn more about the HESI Cardiac Safety Committee
Click to learn more about the Health and Environmental Sciences Institute (HESI)