CT-TRACS & Cell and Gene Therapy Joint Workshop: “Safety Assessment of Cell Therapy Products: Current Advances and Challenges”
- Start Date/Time :
- End Date/Time :
- Location : London, United Kingdom
- Venue : Cell and Gene Therapy Catapult

At this meeting, committee members will:
-Discuss future needs in patient safety of cell-based therapies.
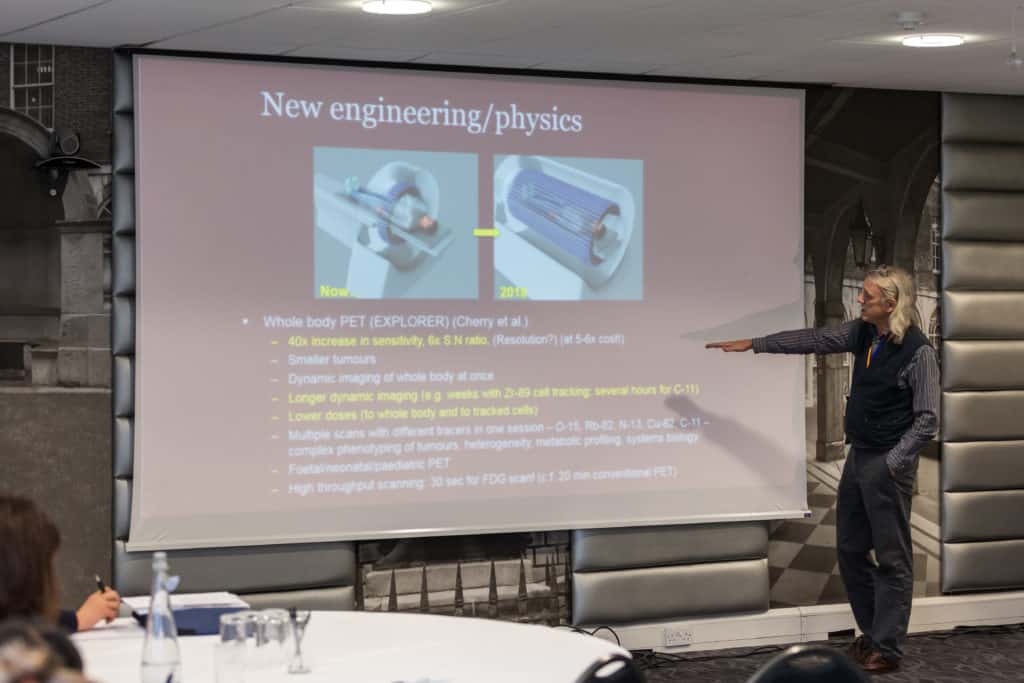
-Learn about evolving safety tools and applied techniques for in vivo cell tracking and tumorigenicity risk evaluation of cell-based products.
-Explore opportunities for international collaborations in cell therapy development and use.
-Network with other industry colleagues in the sector from academia, industry, and regulatory bodies.